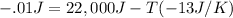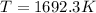Answer:
At 1692.31 K.
Step-by-step explanation:
The Gibbs free energy is a measurement of the energy available for the system to realize a change. And can be calculated by the relation of enthalpy, temperature and entropy by the Gibbs equation:
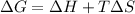
A procces will be spontaneous if the Gibbs free energy of the system is negative.
So, if we set the ΔG of the system as -.1 J to make it so that the proccess will be spontanteous and input the given values of entropy and enthalpy into the Gibbs equation, we get: