Answer: Option (C) is the correct answer.
Step-by-step explanation:
In a chemical reaction, where two different compounds or a mate and a compound are reacting with each other then more reactive metal is able to displace less reactive metal.
When only one element gets displaced then it is known as a replacement or single replacement reaction.
For example,
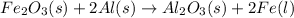
Here, aluminium is more reactive in nature as compared to iron. Hence, iron is replaced by aluminium and this reaction is known as replacement reaction.
Thus, we can conclude that the given chemical reaction is replacement.