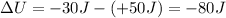Answer:
-80 J
Step-by-step explanation:
The first law of thermodynamics states that:
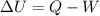
where
 is the change in internal energy of the system
is the change in internal energy of the system
Q is the heat absorbed by the system
W is the work done by the system
In this problem, we have:
Q = -30 J is the heat released by the system (negative because the system releases it)
W = +50 J is the work done by the system on the surrounding (positive since it is done by the system)
Therefore, the change in internal energy is