Answer:
Approximately 3318 disintegrations per minute.
Step-by-step explanation:
The activity
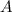 of a radioactive decay at time
of a radioactive decay at time
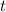 can be found with the following equation:
can be found with the following equation:
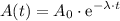 .
.
In this equation,
 is the natural base.
is the natural base.
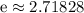 .
.
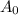 is the initial activity of the decay. For this question,
is the initial activity of the decay. For this question,
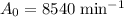 .
.- The decay constant
 of this sample needs to be found.
of this sample needs to be found.
The decay constant here can be found using the activity after 10 days. As long as both times are in the same unit (days in this case,) conversion will not be necessary.
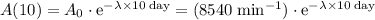 .
.
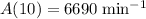 .
.
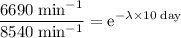
Apply the natural logarithm to both sides of this equation.
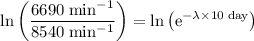 .
.
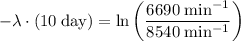 .
.
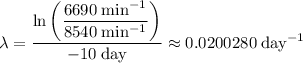 .
.
Note that the unit of the decay constant
 is
is
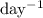 (the reciprocal of days.) The exponent
(the reciprocal of days.) The exponent
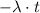 should be dimensionless. In other words, the unit of
should be dimensionless. In other words, the unit of
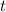 should also be days. This observation confirms that there's no need for unit conversion as long as the two times are in the same unit.
should also be days. This observation confirms that there's no need for unit conversion as long as the two times are in the same unit.
Apply the equation for decay activity at time
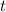 to find the decay activity after 47.2 days.
to find the decay activity after 47.2 days.
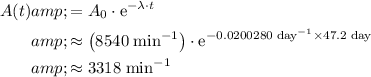 .
.
By dimensional analysis, the unit of activity here should also be disintegrations per minute. The activity after 47.2 days will be approximately 3318 disintegrations per minute.