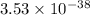Answer :
(a) The value of
 of the lactate ion is,
of the lactate ion is,
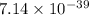
(b) The value of
 of the conjugate base of pyruvic acid is,
of the conjugate base of pyruvic acid is,
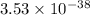
Explanation :
Solution for (a) :
As we are given :
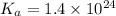
As we know that,
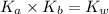
where,
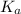 = dissociation constant of an acid =
= dissociation constant of an acid =
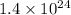
 = dissociation constant of a base = ?
= dissociation constant of a base = ?
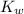 = dissociation constant of water =
= dissociation constant of water =
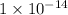
Now put all the given values in the above expression, we get the dissociation constant of a base (lactate ion).
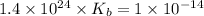
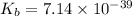
Therefore, the value of
 of the lactate ion is,
of the lactate ion is,
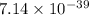
Solution for (b) :
As we are given :
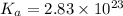
As we know that,
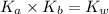
where,
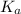 = dissociation constant of an acid =
= dissociation constant of an acid =
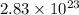
 = dissociation constant of a base = ?
= dissociation constant of a base = ?
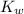 = dissociation constant of water =
= dissociation constant of water =
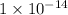
Now put all the given values in the above expression, we get the dissociation constant of a base (conjugate base of pyruvic acid).
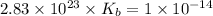
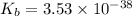
Therefore, the value of
 of the conjugate base of pyruvic acid is,
of the conjugate base of pyruvic acid is,