Answer:
9.2 grams of ethanol should be added.
Step-by-step explanation:
Let the mass of ethanol be x
Density of an ethanol =
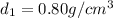
Volume of the ethanol =
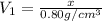
Mass of chloroform = 5.0 g
Density of an chloroform=
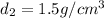
Volume of the ethanol =
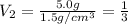
Mass of the mixture,m = x + 5.0 g
Density of the mixture =
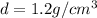
Volume of the mixture=
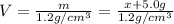
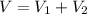
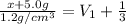
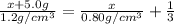
Solving for x , we get
x = 9.2 g
9.2 grams of ethanol should be added.