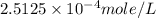Answer : The solubility of nitrogen in water at an atmospheric pressure will be,
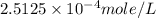
Explanation :
First we have to calculate the partial pressure of nitrogen.
Formula used :
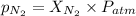
where,
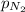 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ?
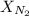 = mole fraction of nitrogen =
= mole fraction of nitrogen =
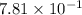
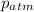 = atmospheric pressure = 0.480 atm
= atmospheric pressure = 0.480 atm
Now put all the given values in the above formula, we get :
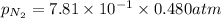
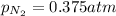
Now we have to calculate the solubility of nitrogen in water.
Formula used :
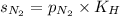
where,
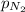 = partial pressure of nitrogen = 0.375 atm
= partial pressure of nitrogen = 0.375 atm
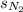 = solubility of nitrogen in water = ?
= solubility of nitrogen in water = ?
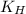 = Henry's constant =
= Henry's constant =
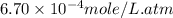
Now put all the given values in the above formula, we get :
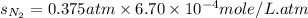
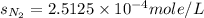
Therefore, the solubility of nitrogen in water at an atmospheric pressure will be,