Answer : The percent yield of the reaction is, 46.49 %
Explanation : Given,
Mass of
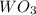 = 74.1 g
= 74.1 g
Molar mass of
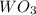 = 231.84 g/mole
= 231.84 g/mole
Molar mass of
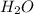 = 18 g/mole
= 18 g/mole
First we have to calculate the moles of
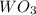 .
.
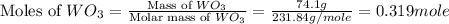
Now we have to calculate the moles of
 .
.
The balanced chemical reaction will be,
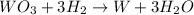
From the balanced reaction, we conclude that
As, 1 mole of
 react to give 3 moles of
react to give 3 moles of
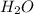
So, 0.319 moles of
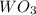 react to give
react to give
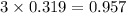 moles of
moles of
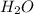
Now we have to calculate the mass of
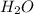
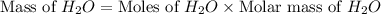
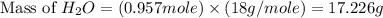
The theoretical yield of
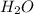 = 17.226 g
= 17.226 g
Now we have to calculate the actual yield of water.
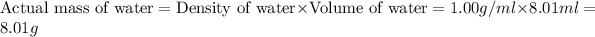
The actual yield of
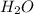 = 8.01 g
= 8.01 g
Now we have to calculate the percent yield of
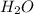
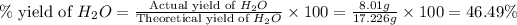
Therefore, the percent yield of the reaction is, 46.49 %