Answer:
The de Broglie wavelength of the helium atoms is
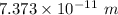 .
.
Step-by-step explanation:
Given that,
Mass
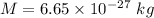
Temperature = 20.0°C
We need to calculate the root-mean square speed
Using formula of root mean square speed
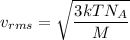
Where, N = Avogadro number
M = Molar mass
T = Temperature
k = Boltzmann constant
Put the value into the formula
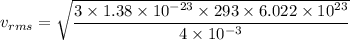
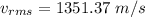
We need to calculate the de Broglie wavelength
Using formula of de Broglie wavelength
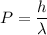
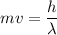
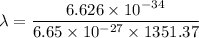
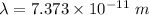
Hence, The de Broglie wavelength of the helium atoms is
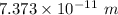 .
.