Answer: 0.736 g
Step-by-step explanation:
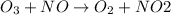
To calculate the moles, we use the equation:

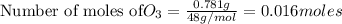
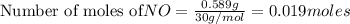
By Stoichiometry:
1 mole of ozone
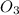 reacts with 1 mole of nitric oxide
reacts with 1 mole of nitric oxide
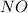 to form 1 mole of nitrogen dioxide
to form 1 mole of nitrogen dioxide
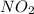
0.016 moles of ozone reacts with=
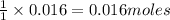 of nitric oxide to form 0.016 mole of
of nitric oxide to form 0.016 mole of
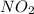
Thus ozone is the limiting reagent as it limits the formation of products and nitric oxide is the excess reagent as (0.019-0.016) g= 0.003 g remains as such.
Mass of
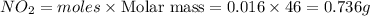
0.736 g of
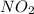 will be produced.
will be produced.