Answer:
The yearly release of
 into the atmosphere is
into the atmosphere is
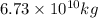 .
.
Step-by-step explanation:
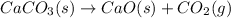
Annual production of CaO =
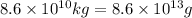
Moles of CaO :
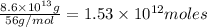
According to reaction, 1 mole of CaO is produced along with 1 mole of carbon-dioxide.
Then along with
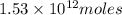 of CaO moles of carbon-dioxide moles produced will be:
of CaO moles of carbon-dioxide moles produced will be:
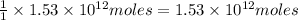 of carbon-dioxide
of carbon-dioxide
Mass of
 moles of carbon-dioxide:
moles of carbon-dioxide:
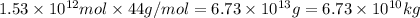
The yearly release of
 into the atmosphere is
into the atmosphere is
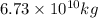 .
.