Answer:
The rate at which dihydrogen is being produced is 0.12 kg/sec.
Step-by-step explanation:
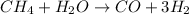 Haber reaction
Haber reaction
Volume of methane consumed in a second = 924 L
Temperature at which reaction is carried out,T= 261°C = 538.15 K
Pressure at which reaction is carried out, P = 0.96 atm
Let the moles of methane be n.
Using an Ideal gas equation:

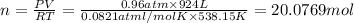
According to reaction , 1 mol of methane gas produces 3 moles of dihydrogen gas.
Then 20.0769 mol of dihydrogen will produce :
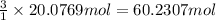 of dihydrogen
of dihydrogen
Mass of 24.3194 moles of ammonia =24.3194 mol × 2 g/mol
=120.46 g=0.12046 kg ≈ 0.12 kg
924 L of methane are consumed in 1 second to produce 0.12 kg of dihydrogen in 1 second. So the rate at which dihydrogen is being produced is 0.12 kg/sec.