Answer:2500-3300
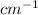
Step-by-step explanation:We can distinguish between 2-butanone and butanoic acid by analyzing the spectra .
The structure of the two compounds are slightly different as butanoic acid has an OH group whereas for 2-butanone there is no OH group present.
Please refer the attachments for structure of compounds.
Both the compounds will show carbonyl stretching frequency at around 1700-1750cm^{-1}[/tex] due to the presence of carbonyl group in both the compounds.
A broad intense peak at around 3000
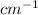 will be observed only for butanoic acid due to the presence of OH group and 2-butanone would not show any broad intense peak at around 3000
will be observed only for butanoic acid due to the presence of OH group and 2-butanone would not show any broad intense peak at around 3000
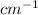 .
.
So by analyzing the IR spectra and identifying the intense broad peak of OH we can easily distinguish between butanoic acid and 2-butanone.
Due to hydrogen bonding in between two carboxylic acid molecules ,peak broadening for OH group in butanoic acid takes place.
Generally the ketones show IR stretching in around 1700-1730cm^{-1}[/tex] due to the presence of carbonyl group.
Generally carboxylic acids show IR stretching for carbonyl group at around 1700-1760cm^{-1}[/tex] and a broad intense peak for OH at around 2500-3000cm^{-1}[/tex].