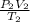Answer:
The ideal gas equation
Step-by-step explanation:
The ideal gas equation is derived from the combination of three gas laws:
- Boyle's law
- Charles's law
- Avogadro's law.
The ideal gas law is expressed mathematically as: PV=nRT where:
P is pressure
V is volume
n is the number of moles
R is the ideal gas law
T is temperature.
To obtain the combined gas law, we assume that n=1 and this gives:
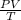 = R
= R
Therefore:
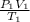 =
=