Answer: B. Pulverizing the zinc metal into a fine powder
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
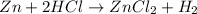
A. Decreasing the concentration of
 and
and
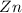 : Thus rate of the reaction would decrease on decreasing the concentration of hydrochloric acid and zinc.
: Thus rate of the reaction would decrease on decreasing the concentration of hydrochloric acid and zinc.
B. Pulverizing the zinc metal into a fine powder : If the solute particles is present in smaller size, more it can take part in the chemical reaction due to large surface area, hence increasing the rate of reaction.
C. Performing the reaction at a lower temperature. Decreasing the temperature means the energy of the particles is less and thus lesser reactants would cross the energy barrier and thus lesser will be the rate.