Answer:
[H+] is decreased.
Step-by-step explanation:
Arrhenius Theory:
An acid is a substance which produces one or more hydrogen ions, (H+) in aqueous solution.
Examples:
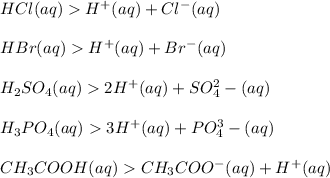
A Base is a substance which produces one or more hydroxyl ion or hydroxide ion (OH-) in aqueous solution.
Examples
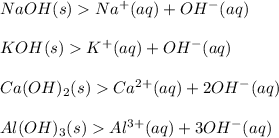
Please note:
(aq) stands for aqueous which means in the presence of water that is, water acts as a solvent
So, on adding a base to the water increase in
![[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xxde7ud270ullhgkdtd9pogar35adf8qg0.png) will take place and this will decrease the Hydrogen ion concentration
will take place and this will decrease the Hydrogen ion concentration
Pure water contains
![[H^+]=[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/8fif0px4qq1zf0vw0kqnv7b6grrykfnzti.png)
if the solution is acidic
![[H^+ ]>[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/td2rwjaxkogn9dkqmg7p5la1hojsi7vycc.png)
if the solution is Basic
![[H^+ ]<[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/wflkjtk5b2lxhn25uo9sskbq195l5hle10.png)