Answer:
Option C
Step-by-step explanation:
As we know that
Relative humidity (RH) is defined as the ratio of amount of water vapor present in a given sample of air to the maximum amount of water that can be absorbed by the same sample of air to attain saturation at a particular temperature .
Thus,
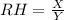
Where X represents the current amount of water vapor present in a given sample of air
and Y represents the maximum amount of water that can be absorbed by the same sample of air to attain saturation at a particular temperature
Substituting the given values in above equation we get -
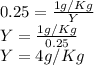
Hence, option C is the correct answer