The correct answer is option E.
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of hydrogen ions concentration in a solution. Mathematically written as:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
The pOH of the solution is defined as negative logarithm of hydroxide ions concentration in a solution. Mathematically written as:
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
The sum of ph and pOH is equal to 14.
pH + pOH = 14
A solution has a pH of 4.20.
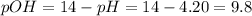
![pOH=9.8=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/tet20npinag0hy1go11vif8p6fgnqvtoa0.png)
![[OH^-]=1.58* 10^(-10) M\approx 6.0* 10^(-10) M](https://img.qammunity.org/2020/formulas/chemistry/high-school/fkmysxjh9bpdhp197ba4frrsnvngjjh5nt.png)
Hence, the correct answer is option E.