Answer:
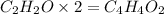 is the molecular formula
is the molecular formula
Step-by-step explanation:
Empirical formula is the simplest form of a Molecular formula.
For example
Molecular formula of glucose is
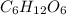
Its empirical formula is
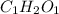
The subscript numbers of the molecular formula is divided by a common number n=6 here.
Another example:
Molecular formula of Octane is
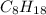 and Its empirical formula will be
and Its empirical formula will be
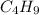
Subscripts divided by n=2.
To find n we make use of the formula
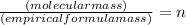
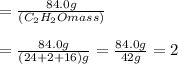
So, we see n=2 here
Empirical formula × n = molecular formula
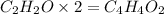 is the molecular formula
is the molecular formula
(Answer)
Please note:
Molar mass is the mass of 1 mole of the substance and its unit is g/mol .
n is the number of moles by which the empirical formula is multiplied to get the molecular formula of the compound.