Answer : The correct option is, kilogram of solvent.
Explanation :
Molality : It is defined as the number of moles of solute present in one kilogram of solvent.
Or, we can say that the number of moles of solute per kilogram of solvent.
The unit of molality is mole per kilogram or mole/Kg.
The formula will be :
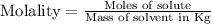
Hence, the molality is a unit of concentration that measures the moles of solute per kilogram of solvent.