Answer:
2.0 atm is the difference between the ideal pressure and the real pressure.
Step-by-step explanation:
If 1.00 mole of argon is placed in a 0.500-L container at 27.0 °C
Moles of argon = n = 1.00 mol
Volume of the container,V = 0.500 L
Ideal pressure of the gas = P
Temperature of the gas,T = 27 °C = 300.15 K[/tex]
Using ideal gas equation:

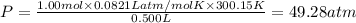
Vander wall's of equation of gases:
The real pressure of the gas=
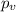
For argon:
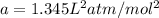
b=0.03219 L/mol.
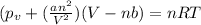
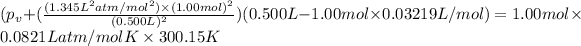
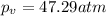
Difference :
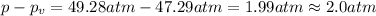
2.0 atm is the difference between the ideal pressure and the real pressure.