Answer:
181 picometers is the radius of a gallium atom.
Step-by-step explanation:
Primitive cubic cell is an arrangement in which constituent particles are present only at the corners of the cube.Also known as simple unit cell. In this arrangement number of total particles is equal to 1.
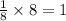
Gallium crystallizes in a primitive cubic unit cell.
Edge length of cubic unit cell = a = 362 pm
Let the radius be gallium atom be r
Two atoms of gallium touching each other = a =2r
362 pm = 2r
r = 181 pm
181 picometers is the radius of a gallium atom.