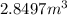Answer: The volume of the container is
Step-by-step explanation:
To calculate the volume of water, we use the equation given by ideal gas, which is:

or,
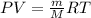
where,
P = pressure of container = 200 kPa
V = volume of container = ? L
m = Given mass of water = 2.61 kg = 2610 g (Conversion factor: 1kg = 1000 g)
M = Molar mass of water = 18 g/mol
R = Gas constant =
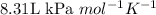
T = temperature of container =
![200^oC=[200+273]K=473K](https://img.qammunity.org/2020/formulas/chemistry/college/35wudhjmjuiic2wm1w2jhcyj66dnrnb1sm.png)
Putting values in above equation, we get:
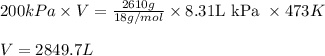
Converting this into cubic meter, we use the conversion factor:
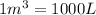
So,
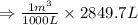
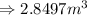
Hence, the volume of the container is