Answer: The volume of the vessel is
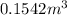 and total internal energy is 162.0 kJ.
and total internal energy is 162.0 kJ.
Step-by-step explanation:
- To calculate the volume of water, we use the equation given by ideal gas, which is:

or,
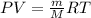
where,
P = pressure of container = 700 kPa
V = volume of container = ? L
m = Given mass of R-134a = 3.98 kg = 3980 g (Conversion factor: 1kg = 1000 g)
M = Molar mass of R-134a = 102.03 g/mol
R = Gas constant =
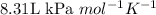
T = temperature of container =
![60^oC=[60+273]K=333K](https://img.qammunity.org/2020/formulas/chemistry/college/xckmaqtnt8yfo4fewmgsxqdrpbuuxkc64g.png)
Putting values in above equation, we get:
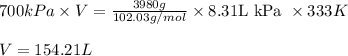
Converting this value into
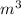 , we use the conversion factor:
, we use the conversion factor:
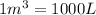
So,
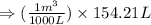
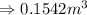
- To calculate the internal energy, we use the equation:
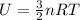
or,
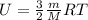
where,
U = total internal energy
m = given mass of R-134a = 3.98 kg = 3980 g (Conversion factor: 1kg = 1000g)
M = molar mass of R-134a = 102.03 g/mol
R = Gas constant =
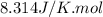
T = temperature =
![60^oC=[60+273]K=333K](https://img.qammunity.org/2020/formulas/chemistry/college/xckmaqtnt8yfo4fewmgsxqdrpbuuxkc64g.png)
Putting values in above equation, we get:
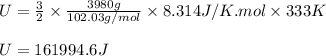
Converting this into kilo joules, we use the conversion factor:
1 kJ = 1000 J
So, 161994.6 J = 162.0 kJ
Hence, the volume of the vessel is
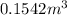 and total internal energy is 162.0 kJ.
and total internal energy is 162.0 kJ.