Answer: The number of moles of nitrogen gas is 9.9 moles.
Step-by-step explanation:
To calculate the mass of bromine gas, we use the ideal gas equation, which is:
PV = nRT
where,
P = Pressure of nitrogen gas = 2.30 atm
V = Volume of nitrogen gas = 120.0 L
n = Number of moles of nitrogen gas = ? mol
R = Gas constant =
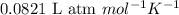
T = Temperature of nitrogen gas = 340 K
Putting values in above equation, we get:
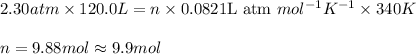
Rule of significant figures in case of multiplication and division:
The least number of significant figures in any number of the problem will determine the number of significant figures in the solution.
Here, the least precise number of significant figures are 2. Thus, the number of moles of nitrogen gas is 9.9 moles.