Answer :
(a) The maximum efficiency of the engine is, 62.5 %
(b) The maximum work done is, 0.625 KJ.
(c) The heat discharge into the cold sink is, 0.375 KJ.
Explanation : Given,
Temperature of hot body
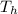 = 800 K
= 800 K
Temperature of cold body
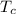 = 300 K
= 300 K
(a) First we have to calculate the maximum efficiency of the engine.
Formula used for efficiency of the engine.
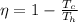
Now put all the given values in this formula, we get :

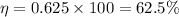
(b) Now we have to calculate the maximum work done.
Formula used :
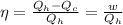
where,
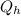 = heat supplied by hot source = 1 KJ
= heat supplied by hot source = 1 KJ
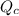 = heat supplied by hot source
= heat supplied by hot source
w = work done = ?
Now put all the given values in this formula, we get :
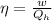
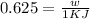
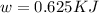
(c) Now we have to calculate the heat discharge into the cold sink.
Formula used :
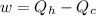
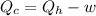
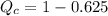
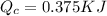
Therefore, (a) The maximum efficiency of the engine is, 62.5 %
(b) The maximum work done is, 0.625 KJ.
(c) The heat discharge into the cold sink is, 0.375 KJ.