Answer : The total change of volume is, 41.883 liters.
Explanation :
R134a is a 1,1,1,2-tetrafluoroethane. It is a hydro-fluorocarbon and haloalkane gaseous refrigerant.
First we have to calculate the volume at
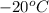 .
.
Using ideal gas equation:
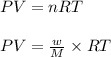
where,
n = number of moles
w = mass of R134a = 257 g
P = pressure of the gas = 60 Kpa
T = temperature of the gas =
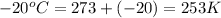
M = molar mass of R134a = 102.03 g/mole
R = gas constant = 8.314 Kpa.L/mole.K
V = initial volume of gas
Now put all the given values in the above equation, we get :
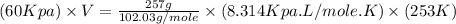
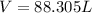
Now we have to calculate the volume at
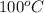 by using Charles's law.
by using Charles's law.
Charles' Law : It is defined as the volume of gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
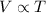
or,
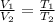
where,
 = initial volume of gas = 88.305 L
= initial volume of gas = 88.305 L
 = final volume of gas = ?
= final volume of gas = ?
 = initial temperature of gas = 253 K
= initial temperature of gas = 253 K
 = final temperature of gas =
= final temperature of gas =
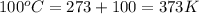
Now put all the given values in the above formula, we get the final volume of the gas.
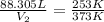
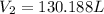
Now we have to calculate the total change of volume.
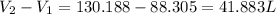
Therefore, the total change of volume is, 41.883 liters.