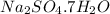Answer:
The formula of the hydrate of the sodium sulfate is :
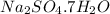
Step-by-step explanation:
Mass of hydrated sodium sulfate = 40.15 gram
Mass of completely dehydrated sodium sulfate = 21.27 gram
Mass of water molecules present in hydrated sodium sulfate =x
40.15 gram = 21.27 gram + x (Law of conservation of mass)
x = 40.15 gram - 21.27 gram = 18.88 g
Moles of water =
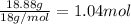
Moles of sodium sulfate =
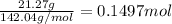
Whole number ratio of sodium sulfate and water:
Sodium sulfate =
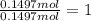
Water =
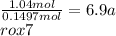
The formula of hydrate be
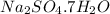
So, the formula of the hydrate of the sodium sulfate is :