Answer:
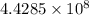 many atoms would it take to make the distance 6.20 cm from end to end.
many atoms would it take to make the distance 6.20 cm from end to end.
Step-by-step explanation:
Diameter of the helium atom =
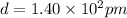
Let the number of atoms required to make the distance 6.20 cm be n.
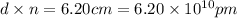
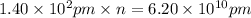
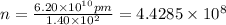
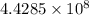 many atoms would it take to make the distance 6.20 cm from end to end.
many atoms would it take to make the distance 6.20 cm from end to end.