Answer:
For a: The mass of one unit cell of copper is
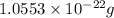
For b: The volume of copper unit cell is
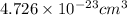
For c: The edge length of the unit cell is
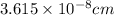
For d: The radius of a copper atom 127.82 pm.
Step-by-step explanation:
We know that:
Mass of copper atom = 63.55 g/mol
According to mole concept:
1 mole of an atom contains
 number of atoms.
number of atoms.
If,
 number of atoms occupies 63.55 grams.
number of atoms occupies 63.55 grams.
So, 1 atom will occupy =
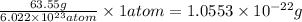
Hence, the mass of one unit cell of copper is
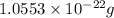
Copper crystallizes with a face-centered cubic lattice. This means that 4 number of copper atoms are present in 1 units cell.
Mass of 4 atoms of copper atom =
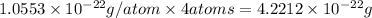
We are given:
Density of copper =
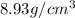
To find the volume of copper, we use the equation:
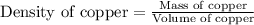
Putting values in above equation, we get:
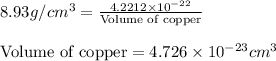
Hence, the volume of copper unit cell is
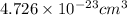
Edge length of the unit cell is taken as 'a'
Volume of cube =
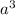
Putting the value of volume of unit in above equation, we get:
![\sqrt[3]{4.726* 10^(-23)}cm^3=3.615* 10^(-8)cm](https://img.qammunity.org/2020/formulas/chemistry/college/nofj4lj11ct4cvzecowwudxks1t65as4yr.png)
Hence, the edge length of the unit cell is
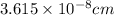
The relation of radius and edge length for a face-centered lattice follows:
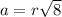
Putting values in above equation, we get:
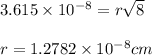
Converting cm to pm, we get:
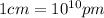
So,
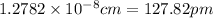
Hence, the radius of a copper atom 127.82 pm.