Answer: The equilibrium concentration of
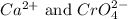 are 0.0266 M.
are 0.0266 M.
Step-by-step explanation:
The chemical equation for the ionization of calcium chromate follows:
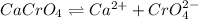
The expression for equilibrium constant is given as:
![K_c=([Ca^(2+)][CrO_4^(2-)])/([CaCrO_4])](https://img.qammunity.org/2020/formulas/chemistry/college/6mvgq0swj1gmzc0c60oa3syoq3r62njov5.png)
We are given:
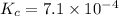
The concentration of solid substances are taken to be 1. Thus, they do not appear in the equilibrium constant expression.
Let the equilibrium concentration for
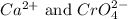 be 'x'
be 'x'
Putting values in above equation, we get:
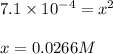
Hence, the equilibrium concentration of
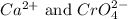 are 0.0266 M.
are 0.0266 M.