Answer:
The final temperature of the system is 16.4°C.
Step-by-step explanation:
Given that,
Mass of ice cube = 0.0811 kg=81.1 g
Mass of water = 0.397 kg = 397 g
Initial temperature of ice cube = 0°C=273 K
Initial temperature of water = 14.8°C = 14.8+273=287.8 K
We need to calculate the final temperature
We know that,
Specific capacity of solid = 2.09 J/g°C
Using formula of energy
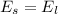
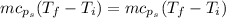
Put the value into the formula
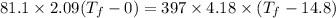
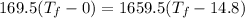
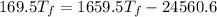
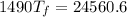
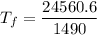
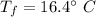
Hence, The final temperature of the system is 16.4°C.