Answer : The mass of nitrogen present are, 4.965 grams.
Explanation :
According to the Raoult's law,
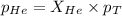
where,
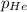 = partial pressure of gas = 231 mmHg
= partial pressure of gas = 231 mmHg
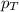 = total pressure of gas = 641 mmHg
= total pressure of gas = 641 mmHg
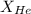 = mole fraction of helium gas = ?
= mole fraction of helium gas = ?
Now put all the given values in this formula, we get the mole fraction of helium gas.
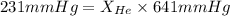
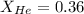
Now we have to calculate the mole fraction of nitrogen gas.
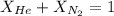
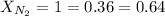
Now we have to calculate the mass nitrogen gas.
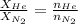
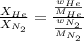
where,
n = moles, w = mass, M = molar mass
Now put all the given values in this expression, we get:
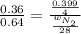
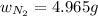
Therefore, the mass of nitrogen present are, 4.965 grams.