Answer:
The change in internal energy of the system is -17746.78 J
Step-by-step explanation:
Given that,
Pressure
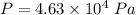
Remove heat
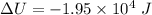
Radius = 0.272 m
Distance d = 0.163 m
We need to calculate the internal energy
Using thermodynamics first equation
 ...(I)
...(I)
Where, dU = internal energy
Q = heat
W = work done
Put the value of W in equation (I)
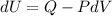
Where, W = PdV
Put the value in the equation
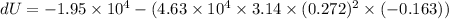
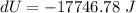
Hence, The change in internal energy of the system is -17746.78 J