Answer: 0.07 moles
Step-by-step explanation:
According to Dalton's law, the total pressure of a mixture of gases is the sum of individual pressures exerted by the constituent gases.
Thus
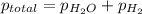
Given:
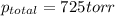
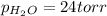
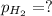
Thus
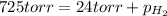
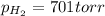
According to the ideal gas equation:'

P= Pressure of the gas = 701 torr = 0.92 atm (760torr=1atm)
V= Volume of the gas = 1.85 L
T= Temperature of the gas = 308 K
R= Value of gas constant = 0.082 Latm\K mol
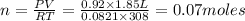
Thus 0.07 moles of hydrogen gas is contained in 1.85 L of this mixture at 308 K.