Answer : The molality, mass/mass percent, and mass/volume percent are, 0.0381 mole/Kg, 25.67 % and 32.086 % respectively.
Solution : Given,
Density of solution = 1.25 g/ml
Molar mass of
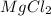 (solute) = 95.21 g/mole
(solute) = 95.21 g/mole
3.37 M magnesium chloride means that 3.37 gram of magnesium chloride is present in 1 liter of solution.
The volume of solution = 1 L = 1000 ml
Mass of
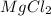 (solute) = 3.37 g
(solute) = 3.37 g
First we have to calculate the mass of solute.
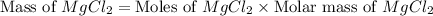
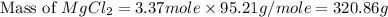
Now we have to calculate the mass of solution.
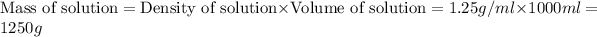
Mass of solvent = Mass of solution - Mass of solute = 1250 - 320.86 = 929.14 g
Now we have to calculate the molality of the solution.
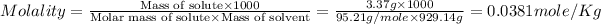
The molality of the solution is, 0.0381 mole/Kg.
Now we have to calculate the mass/mass percent.
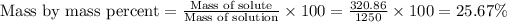
The mass/mass percent is, 25.67 %
Now we have to calculate the mass/volume percent.
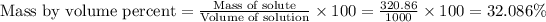
The mass/volume percent is, 32.086 %
Therefore, the molality, mass/mass percent, and mass/volume percent are, 0.0381 mole/Kg, 25.67 % and 32.086 % respectively.