Answer:
27.77 kJ/mol is the activation energy for the reaction.
Step-by-step explanation:
Rate of the reaction at 323 K =
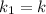
Rate of the reaction at 373 K =
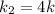
Activation energy for the reaction is calculated by formula:
![\log (k_2)/(k_1)=(E_a)/(2.303* R)[(T_2-T_1)/(T_2* T_1)]](https://img.qammunity.org/2020/formulas/chemistry/college/reinqt025ieno19k69vsln8ac61epj5qw6.png)
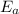 = Activation energy
= Activation energy
 = Temperature when rate of the reaction was
= Temperature when rate of the reaction was
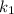
 = Temperature when rate of the reaction was
= Temperature when rate of the reaction was
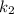
Substituting the values:
![\log (4k)/(k)=(E_a)/(2.303* 8.314 J /mol K)[(373 K-323K)/(373 K* 323 K)]](https://img.qammunity.org/2020/formulas/chemistry/college/5vgrqtb5zs100owbya0ejtqol5ykxodid6.png)
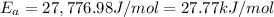
27.77 kJ/mol is the activation energy for the reaction.