Answer:
The annual production of carbon dioxide is
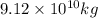 .
.
Step-by-step explanation:
Distance covered by each car = 5790 miles
Rate of consumption of gasoline =24. mile/gal
For every 24.1 mile 1 gallon of gasoline is used
Gasoline used by a single car by travelling 5790 miles =
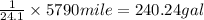
Number of cars in the United states = 40.0 million =
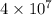
Total gallons of gasoline consumed by 40 million cars =
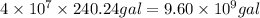
1 gallon of gasoline produces = 9.50 kg of

Then
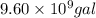 of gasoline will produce:
of gasoline will produce:
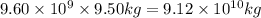 of
of

The annual production of carbon dioxide is
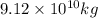 .
.