Answer: The empirical formula of compound is
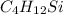 .
.
Step-by-step explanation:
Mass of Sample= 0.702 g
Mass of
 = 1.4 g
= 1.4 g
Mass of
 = 0.86 g
= 0.86 g
Mass of
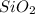 = 0.478 g
= 0.478 g
First we have to calculate moles of
 ,
,
 and
and
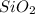 formed.
formed.
1. Moles of
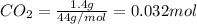
Now , Moles of carbon == Moles of
 = 0.032
= 0.032
2. Moles of
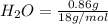 =0.048mol
=0.048mol
Now , Moles of hydrogen =
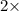 Moles of
Moles of
 =
=
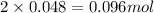
3. Moles of
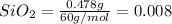 mol
mol
Now , Moles of silicon = Moles of
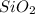 = 0.008 moles
= 0.008 moles
Therefore, the ratio of number of moles of C : H : Si is = 0.032 : 0.096 : 0.008
For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For C=
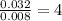
For H =
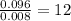
For Si=
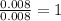
Thus, C: H: Si = 4 : 12 : 1
The simplest ratio represent empirical formula.
Hence, the empirical formula of compound is
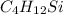 .
.