Step-by-step explanation:
a) Manganese (II) phosphite :
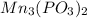
Number of phosphite ions in manganese (II) phosphite =

In one molecule of manganese (II) phosphite there are 2 ions of phosphite.
Then
 ions of phosphite will be ions will be in:
ions of phosphite will be ions will be in:
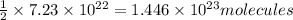
Moles of manganese (II) phosphite;=
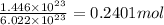
Mass of 0.2401 mol of Manganese (II) phosphite :
0.2401 mol × 322.75 g/mol = 77.49 g
77.49 is the mass in grams of a sample of manganese (II) phosphite.
b) Molar mass of ammonium chromate =252.07 g/mol
Percentage of Nitrogen:
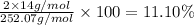
Percentage of hydrogen:
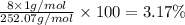
Percentage of chromium:
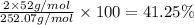
Percentage of oxygen:
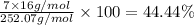
c) Molar mass of the substance = 202.23 g/mol
Percentage of the hydrogen = 4.98 %
Let the molecular formula be
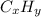
Percentage of the carbon = 95.02 %
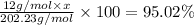
x= 16.01 ≈ 16
Percentage of the hydrogen= 4.98 %
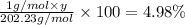
y= 10.07 ≈ 10
The molecular formula of the substance is
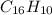 .
.
The empirical formula of the substance is
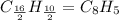 .
.