Answer: The concentration of
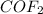 remains at equilibrium is 0.40 M.
remains at equilibrium is 0.40 M.
Explanation:-
Initial concentration of
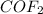 = 2 M
= 2 M
The given balanced equilibrium reaction is,
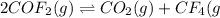
Initial conc. 2 M 0 0
At eqm. conc. (2-2x) M x M x M
The expression for equilibrium constant for this reaction will be,
![K_c=([CO_2]* [CF_4])/([COF_2]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/ewymyc6d91dolh7mnkm9n33df6nqq4dzg4.png)
Now put all the given values in this expression, we get :
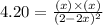
By solving the term 'x', we get :
x = 0.80 M
Thus, the concentrations of
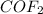 at equilibrium is : (2-2x) = 2-2(0.80)=0.40 M
at equilibrium is : (2-2x) = 2-2(0.80)=0.40 M
The concentration of
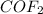 remains at equilibrium is 0.40 M.
remains at equilibrium is 0.40 M.