Answer:
A) 854.46 kPa
Step-by-step explanation:
P₁ = initial pressure of the gas = 400 kPa
P₂ = final pressure of the gas = ?
T₁ = initial temperature of the gas = 110 K
T₂ = final temperature of the gas = 235 K
Using the equation
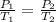
Inserting the values
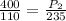
P₂ = 854.46 kPa