Answer:
Heat energy needed = 1243.45 kJ
Step-by-step explanation:
We have
heat of fusion of water = 334 J/g
heat of vaporization of water = 2257 J/g
specific heat of ice = 2.09 J/g·°C
specific heat of water = 4.18 J/g·°C
specific heat of steam = 2.09 J/g·°C
Here wee need to convert 0.500 kg water from 50°C to vapor at 110°C
First the water changes to 100°C from 50°C , then it changes to steam and then its temperature increases from 100°C to 110°C.
Mass of water = 500 g
Heat energy required to change water temperature from 50°C to 100°C
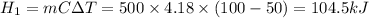
Heat energy required to change water from 100°C to steam at 100°C

Heat energy required to change steam temperature from 100°C to 110°C
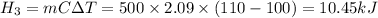
Total heat energy required
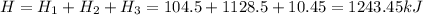
Heat energy needed = 1243.45 kJ