Answer:
D) "The reaction will shift in the direction of the reactants."
Step-by-step explanation:
Consider the Le Chatelier's Principle. When there's a change in the conditions of an equilibrium, the equilibrium will shift in a way that minimizes the impact of those changes.
In this question, the change is an increase in the partial pressure of
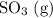 (analog to concentration in a solution.)
(analog to concentration in a solution.)
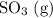 is a product of the forward reaction and is consumed in the reverse reaction. Shifting the equilibrium towards the reactants will consume some of the additional
is a product of the forward reaction and is consumed in the reverse reaction. Shifting the equilibrium towards the reactants will consume some of the additional
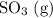 and reduce its partial pressure.
and reduce its partial pressure.
Alternatively, think about equilibrium as a balance between the forward and the backward reaction. When the system is at equilibrium, the two reaction rates are equal, so overall the composition will stay the same. However, when more of the product
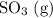 is suddenly added to the system, the rate of the reverse reaction will jump upwards while the rate of the forward reaction will only gradually increase. Before the system reach a new equilibrium position, the reverse reaction will prevail and shift the equilibrium towards the reactants.
is suddenly added to the system, the rate of the reverse reaction will jump upwards while the rate of the forward reaction will only gradually increase. Before the system reach a new equilibrium position, the reverse reaction will prevail and shift the equilibrium towards the reactants.
Either way, adding the product
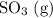 to the system will shift the equilibrium towards the reactants. The Le Chatelier's principle might be easier to memorize. However, keep in mind that the Le Chatelier's principle is only a generalization of the observations; only the second explanation describes what's actually going on in the equilibrium.
to the system will shift the equilibrium towards the reactants. The Le Chatelier's principle might be easier to memorize. However, keep in mind that the Le Chatelier's principle is only a generalization of the observations; only the second explanation describes what's actually going on in the equilibrium.
Choice B) is not what will happen since there's an equal number of gas particles on both sides of this reaction. If all four gases behave like ideal gases, the shift in equilibrium position will not change the pressure if temperature stays the same.
As a side note on choice E), for a certain reaction, the equilibrium constant depends only on the temperature. In other words, adding or removing a reactant or a product will not change the equilibrium constant.