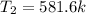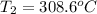Answer:
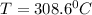
Step-by-step explanation:
Here by ideal gas equation we can say
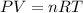
now we know that pressure is kept constant here
so we will have
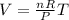
since we know that number of moles and pressure is constant here
so we have
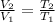
now we know that initial temperature is 17.8 degree C
and finally volume is doubled
So we have
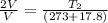
so final temperature will be