Answer : The DePriester K-value for the species in the mixture is, 0.4
Explanation :
According to the Dalton's Law, the partial pressure exerted by component 'i' in a gas mixture is equal to the product of the mole fraction of the component and the total pressure.
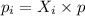 ........(1)
........(1)
According to the Raoult's law, the partial pressure exerted in gas phase by a component is equal to the product of the vapor pressure of that component and its mole fraction for an ideal liquid solution.
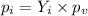 ........(2)
........(2)
When the gas and the liquid are in equilibrium then these partial pressures must be the same.
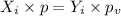
or,
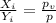
This ration is called as equilibrium ratio
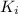 of the i-th component.
of the i-th component.
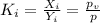 .......(3)
.......(3)
As we are given that,
Total pressure =
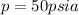
The vapor pressure =
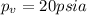
According to the relation (3), we get
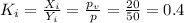
Therefore, the DePriester K-value for the species in the mixture is, 0.4