Answer: The fugacity coefficient of a gaseous species is 1.25
Step-by-step explanation:
Fugacity coefficient is defined as the ratio of fugacity and the partial pressure of the gas. It is expressed as
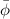
Mathematically,
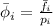
Partial pressure of the gas is expressed as:
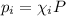
Putting this expression is above equation, we get:
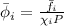
where,
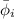 = fugacity coefficient of the gas
= fugacity coefficient of the gas
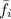 = fugacity of the gas = 25 psia
= fugacity of the gas = 25 psia
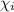 = mole fraction of the gas = 0.4
= mole fraction of the gas = 0.4
P = total pressure = 50 psia
Putting values in above equation, we get:
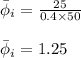
Hence, the fugacity coefficient of a gaseous species is 1.25