Answer:
final equilibrium temperature of the system is ZERO degree Celcius
Step-by-step explanation:
Hear heat given by water + iron = heat absorbed by ice
so here first we will calculate the heat given by water + iron
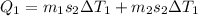
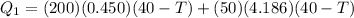
now the heat absorbed by ice so that it will melt and come to the final temperature
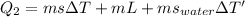
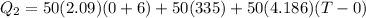
now we will have
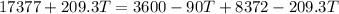
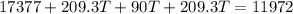

since it is coming out negative which is not possible so here the ice will not completely melt
so final equilibrium temperature of the system is ZERO degree Celcius