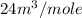Answer : The molar volume of the solution is,
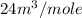
Explanation : Given,
Partial molar volumes of component A =
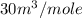
Partial molar volumes of component B =
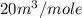
Mole fraction of component A = 0.4
First we have to calculate the mole fraction of component B.
As we know that,
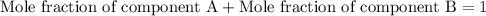
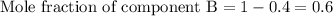
Now we have to calculate the molar volume of the solution.
Expression used :
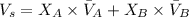
where,
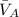 = partial molar volumes of component A
= partial molar volumes of component A
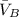 = partial molar volumes of component B
= partial molar volumes of component B
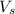 = molar volume of the solution
= molar volume of the solution
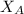 = mole fraction of of component A
= mole fraction of of component A
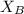 = mole fraction of of component B
= mole fraction of of component B
Now put all the give values in the above expression, we get:
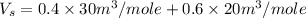
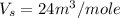
Therefore, the molar volume of the solution is,