Answer:
8.75 grams of tolnaftate powder is needed.
Step-by-step explanation:
Mass by mass percentage (w/w) % : It is the percentage of solute present in 100 grams of solution.
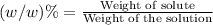
Given mass of the solution = 125 g
Mass of the solute required = x
Mass by mass percentage of the solution = 7%
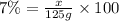
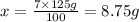
8.75 grams of tolnaftate powder is needed.